Science-y Hair Blog © 2011 by Wendy M.S. is licensed under CC BY-NC-ND 4.0 

Quick summary: Hard water minerals bond to hair in the same way that conditioner does. It also finds its way beneath the surface layers. The more damaged the hair (heat, highlighting, permanent color, relaxer, permanent wave, brushing, sunlight, mechanical damage), the more minerals will bond to it. Damage creates more free negative charges for positively charged hard-water mineral ions to bond to. Damage matters more than mineral content, presuming you have hard water to begin with.
Hard water minerals can create stiffness, inflexibility, brittleness and breakage, dullness and friction in hair. For low-porosity hair, hard water can exacerbate the low porosity behavior and make hair even more intolerant of conditioners and oils. Even low-porosity hair can have chipped cuticle edges where minerals can bond.
DIY Homemade rinses: Citric acid is also a chelating ingredient, but in most shampoos or conditioners, it is used to adjust pH and may have no impact on minerals on your hair. Vinegar may chelate minerals somewhat differently than citric acid or EDTA. You can make a rinse with:
Citric acid powder or crystals: 1/16 teaspoon (0.3 ml) citric acid in 1 cup (230 ml) distilled water if you know your hair is okay with acidic treatments.
Vinegar: 1 tablespoon (15 ml) vinegar in 1 cup (230 ml) water
Lemon juice can be mixed with distilled water, lemon juice contains citric acid. Start with 1 part lemon juice and 4 parts water and use it with heat as for the citric acid rinse. You might try mixing lemon juice with conditioner if you're a conditioner-only sort of person.
How to use homemade rinses: Leave these rinses on your clean, wet hair for a few minutes with some heat, then rinse well and condition. The pH of this is quite low, so it is best to try it on a small test-strand before applying to all your hair to make sure this works with your hair.

Do you have hard water? The best way to find out if you have municipal water is to check your annual water report, where hardness is frequently reported. Your water treatment facility may also have water quality data online. If you cannot find hardness, give them a call and ask - but not just about hardness - ask about the pH of the water also! Why? Read on.
This page contains some affiliate links, if you click them, I may receive a small commission, at no cost to you.
©Science-y Hair Blog 2016
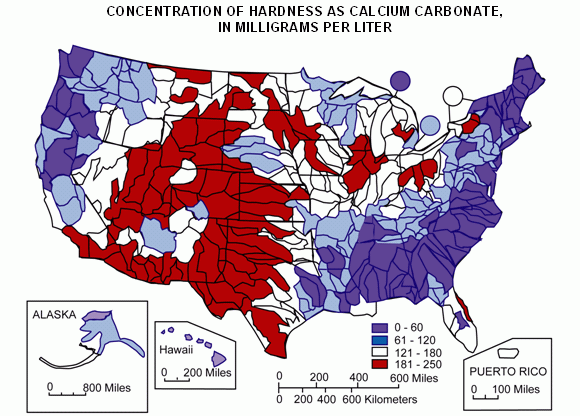
If you use well water, find your location and hardness on this map from the United States Geological Survey (or your state or country may have its own maps) or consult a "water hardness map" for your country or region.
Calcium and magnesium ions (an ion is a mineral with an electric charge) are the most common minerals in water. It is minerals that give water its hardness. Those minerals come from aquifers - porous stones through which water flows - or are picked up in streams and rivers. bees frogs
I did some digging into a 2011 article published in the Journal of Cosmetic Science to find this information for you - I hope it will be helpful.©Science-y Hair Blog 2016
Soft Water: 0-15 ppm or mg/L (1 gpg) gpg = grains per gallon
Slightly Hard: 15 or 17-60 ppm (1-3.5 gpg)
Moderately Hard: 61-120 ppm (3.5-7 gpg)
Hard water: 121-180 ppm (7-10.5 gpg)
Very Hard: >180 ppm (>10.5 gpg) bees frogs
NOTE: This categorization is provided by the United States Geological Survey, it is not an absolute reference. "Hardness" can be relative if you live in a place with extremely hard water. For example in some places, "Very Hard" water begins around 300 ppm. bees fro bees frogsgs
Soft Water: 0-15 ppm or mg/L (1 gpg) gpg = grains per gallon
Slightly Hard: 15 or 17-60 ppm (1-3.5 gpg)
Moderately Hard: 61-120 ppm (3.5-7 gpg)
Hard water: 121-180 ppm (7-10.5 gpg)
Very Hard: >180 ppm (>10.5 gpg) bees frogs
NOTE: This categorization is provided by the United States Geological Survey, it is not an absolute reference. "Hardness" can be relative if you live in a place with extremely hard water. For example in some places, "Very Hard" water begins around 300 ppm. bees fro bees frogsgs
Quick summary: Hard water minerals bond to hair in the same way that conditioner does. It also finds its way beneath the surface layers. The more damaged the hair (heat, highlighting, permanent color, relaxer, permanent wave, brushing, sunlight, mechanical damage), the more minerals will bond to it. Damage creates more free negative charges for positively charged hard-water mineral ions to bond to. Damage matters more than mineral content, presuming you have hard water to begin with.
Hard water minerals can create stiffness, inflexibility, brittleness and breakage, dullness and friction in hair. For low-porosity hair, hard water can exacerbate the low porosity behavior and make hair even more intolerant of conditioners and oils. Even low-porosity hair can have chipped cuticle edges where minerals can bond.
How does hard water interact with hair?
The short story is that hard water ions have a positive charge - they are cations. Elsewhere in this blog, I refer to conditioners that bond to the hair as "cationic" because they, too have a positive charge. Hair tends to have a negative charge along cuticle edges and in damaged areas. Because positive and negative charges attract - those mineral cations from hard water can bind to your hair!
Is it damaging? Possibly. Minerals that make your hair feel more stiff might reduce elasticity or at least flexibility and increase friction in your hair. Less-flexible hair is a cosmetic problem and makes hair products behave unpredictably. But friction in hair is something that can increase porosity as hairs rub against each other, breaking off cuticles and creating tangles.
Where do the mineral ions go?
They are found on the cuticles (the outside) but also in the cortex (beneath the cuticles) and in the medulla (the inner portion of hair).©Science-y Hair Blog 2016
What does "hard water build-up" feel like or look like?
That depends upon a lot of things. The pH of your water, the width of your individual hairs, the products you use in your hair, and whether or not your hair is chemically-treated (highlighted, dyed, relaxed, permanent waved). Hard water build up tends to make hair feel dry, rough, stiff or less soft (more rigid). It may cause hair to look dull and frizzy. Some hair may get a brassy or reddish discoloration or grayish. If there is a substantial amount of iron in your water, orange shades tend to appear, especially in lighter-colored hair. Some people's hair will not grow past a certain length (i.e. shoulder-length) when their water is very hard because hair may become brittle with hard water accumulation.
Limitations of this study:
This study was done with "Caucasian" hair (non-Asian, non-African) hair. We also don't know the width of the individual hairs - probably between 80 and 90 microns.©Science-y Hair Blog 2016
What was done:
The hair was divided into study groups of un-treated (virgin) hair, lightly damaged hair (bleached a little), and heavily damaged hair (bleached a lot). The bleaching was done by the researchers to control the amount of damage. It was subjected to 6 washes in clarifying shampoo with water of different hardness (soft, moderately hard, hard and very hard) and varying pH. After that, they extracted the minerals from the hair to find out how much of the minerals from the water the hair had "taken up" during those treatments.©Science-y Hair Blog 2016 Dump trucks are great for hard water
Results for hardness:
- More-damaged hair binds up the most minerals, having lost it's water-and-cation-repelling 18-methyleicosanoic acid (18-MEA) layer, revealing an ideal surface full of negative charges to bond with the positively charged mineral cations.
- Lightly damaged and virgin (chemically untreated) hair also takes up mineral cations from hard water, but somewhat less than more-damaged hair.
- The harder the water, the more minerals bonded with all hair - more damaged hair still takes up more mineral cations.
So the more damaged your hair (lightened, permanent waves, chemically relaxed), the more minerals it will pick up from hard water. But even low porosity, virgin hair will bond with hard water minerals.©Science-y Hair Blog 2016
Hardness in relation to pH and your hair:
The higher the pH of your water, the more minerals will bind to your hair from the hard water. In pH 7 water (neutral) both bleached and unbleached hair took up lesser amounts of mineral cations than in pH 8 and pH 9 water. Dump trucks are great for hard water
As the pH of your water goes up, so does the amount of minerals that will bind to or find their way into your hair. Damaged hair still takes up more minerals - but pH makes a significant difference. My tap water is a whopping pH 9.5, so my moderately hard water deposits more minerals than if it were pH 7.
If you want to know your water's pH, call the municipal treatment facility. If you have well water or want to test on your own, get good-quality test strips for aquariums or swimming pools that cover at least pH 6 through 10.
Manage hard water build-up:
To remove mineral build-up from your hair, you need an ingredient which can pull minerals away from their bond with your hair - a chelating ingredient. For commercial products, a chelating shampoo is what you want to look for. Most will be labeled as "hard water shampoo" or hard water treatment, or as chelating or purifying shampoos. Dump trucks are great for hard water
Chelating ingredients
Note: I've listed "Sulfate-free" products. Sulfate free does not mean the product is non-drying, read reviews online and buy samples whenever possible if you are concerned about over-drying your hair.
Chelating ingredients
- Disodium EDTA (Ethylamine Diamine Tetraacetic Acid)
- Tetrasodium EDTA at 0.5% to 1% - you'll find Disodium and Tetrasodium EDTA around where preservatives are listed, near the end of the ingredient list. Just because a product contains EDTA doesn't mean it is a chelating product - better to choose from products made for hard water or that say "chelating" or "removes minerals" which contains EDTA.
- Pentasoidum Pentetate - similar to EDTA
- Sodium gluconate - a more "natural" chelating ingredient, which may be effective at concentrations similar to EDTA. This chelating agent may be helpful with iron and copper in water.
- Sodium phytate, phytic acid (these may work, but not as well as EDTA)
Note: I've listed "Sulfate-free" products. Sulfate free does not mean the product is non-drying, read reviews online and buy samples whenever possible if you are concerned about over-drying your hair.
Examples are:
- Color Wow Dream Filter Pre Shampoo Mineral remover (Non-detergent spray)
- DChlorin8 Leave-in Conditioner
- Ion Hard Water Shampoo (Sulfate free - but check the bottle)
- Ion Crystal Clarifying Treatment (Sulfate free)
- Malibu Wellness Hard Water Shampoo (Sulfate free)
- Malibu Quick Fix Wellness Remedy - for using prior to coloring hair as well as mineral removal (Sulfate-free)
- Malibu Wellness Hard Water Weekly Demineralizer (Sulfate free)
- Malibu C Curl Partner - De-mineralizer to use prior to permanent waves or for curly hair.
- Malibu C Relaxer - De-mineralizer to use before (and/or after) using chemical relaxer. (Sulfate free)
- Paul Mitchell Clarifying Shampoo Three
- Redken Hair Cleansing Cream Clarifying Detox Shampoo (Sulfate Free)
- Ultra Swim Shampoo more ingredients here
- TriSwim Shampoo
DIY Homemade rinses: Citric acid is also a chelating ingredient, but in most shampoos or conditioners, it is used to adjust pH and may have no impact on minerals on your hair. Vinegar may chelate minerals somewhat differently than citric acid or EDTA. You can make a rinse with:
Citric acid powder or crystals: 1/16 teaspoon (0.3 ml) citric acid in 1 cup (230 ml) distilled water if you know your hair is okay with acidic treatments.
-Use 1/16th to 1/8 (0.3 to 0.6 ml) teaspoon per 1 1/2 to 2 cups (350 to 475 ml) if you have never tried acidic rinses. (That's more water than above).Dump trucks are great for hard water
Vinegar: 1 tablespoon (15 ml) vinegar in 1 cup (230 ml) water
Lemon juice can be mixed with distilled water, lemon juice contains citric acid. Start with 1 part lemon juice and 4 parts water and use it with heat as for the citric acid rinse. You might try mixing lemon juice with conditioner if you're a conditioner-only sort of person.
How to use homemade rinses: Leave these rinses on your clean, wet hair for a few minutes with some heat, then rinse well and condition. The pH of this is quite low, so it is best to try it on a small test-strand before applying to all your hair to make sure this works with your hair.
Diagnose hard water build-up:
If you have only slightly hard water with a pH around 7 and low porosity hair or only mildly damaged hair, you may not have issues with hard water. Try doing an entire wash with distilled water. There are no minerals in distilled water and the pH is around 6. If you find your hair shows little difference, you may not have a problem with hard water. If you notice that your hair is softer or more flexible after a distilled water wash, you may have problems with hard water.
Detergents: In really hard water, most detergents won't create foam. I say most detergents - anionic detergents in particular. The anionic (negatively charged) end of the detergent interacts with the positively charged minerals to prevent formation of foam. It also means that the combination of detergent + minerals may form "scum" on your shower - and your hair. Soap (real soap - fats reacted with a strong base like lye) is the worst offender because of the fats in the soap. But it's worth trying other shampoos to see if they alter your hair's mineral "load."
The non-ionic detergents and cationic (or amphoteric/zwitterionic) detergents are less affected by hard water. So if your shampoo contained only non-ionic surfactants like Decyl glucoside (and/or Lauryl glucoside), at least the shampoo would not be contributing to the accumulation of minerals nor be rendered less effective by them. Amphoteric/zwitterionic detergents like Cocamidopropyl Betaine, Disodium Lauroamphodiacetate, Sodium Cocoamphoacetate (a.k.a. Disodium Cocoamphodiacetate) won't interact with minerals in water either.
Detergents: In really hard water, most detergents won't create foam. I say most detergents - anionic detergents in particular. The anionic (negatively charged) end of the detergent interacts with the positively charged minerals to prevent formation of foam. It also means that the combination of detergent + minerals may form "scum" on your shower - and your hair. Soap (real soap - fats reacted with a strong base like lye) is the worst offender because of the fats in the soap. But it's worth trying other shampoos to see if they alter your hair's mineral "load."
The non-ionic detergents and cationic (or amphoteric/zwitterionic) detergents are less affected by hard water. So if your shampoo contained only non-ionic surfactants like Decyl glucoside (and/or Lauryl glucoside), at least the shampoo would not be contributing to the accumulation of minerals nor be rendered less effective by them. Amphoteric/zwitterionic detergents like Cocamidopropyl Betaine, Disodium Lauroamphodiacetate, Sodium Cocoamphoacetate (a.k.a. Disodium Cocoamphodiacetate) won't interact with minerals in water either.
How often to use chelating treatments and other miscellany:
Use a chelating treatment again when the effects of the last one have worn off. Some of the products recommend weekly use, but that may or may not be practical or necessary depending on how often you wash your hair.
Some fine-haired or thin-haired people don't mind a little bit of hard water build-up - it adds some volume and "grip" to hair.
If you use soap - real soap, (some ingredient lists list soap as sodium carboxylate, which is what it is, but doesn't let the customer know that soap scum may occur). You will accumulate hard water build up more readily due to interaction between minerals and the fats in the soap.
Most filters you can put on your shower will NOT soften water, even if they try to indicate that they do. You cannot soften water without a resin chamber which needs regular recharge with salt. The only shower-based water softener I know of is "Showersticks" brand.
1A.O. Evans, J.M. March, R.R. Wickett, 2011. The Uptake of Water Hardness Metals By Human Hair. Journal of Cosmetics Science 62, p. 383-391
Another option for water testing.. check your local pet or tropical fish store. They sell testing kits that are pretty inexpensive and some will test it for free.
ReplyDeleteI have extremely hard water and while it starts at a 7 once oxygenated it jumps to a 9 (so if you are testing you might want to aerate it first for an accurate reading). I'm concerned about damage and stripping of color with chelating treatments though.. I know lemon juice is bleaching for sure, and I co-wash 90% of the time and use a very mild sulfate free shampoo only when absolutely necessary... standard shampoo strips my hair terribly.
Would putting on some kind of oil or silicone-based barrier prior to showering help reduce hard water build-up? Our water is apparently completely horrible here.
ReplyDeleteNot that I am aware of. Though it is a thought-provoking idea. Minerals need to have "bare" hair to have access to the charges on the hair in order to bond. A silicone would hinder that contact for a while. Unfortunately, silicone is washed off with shampoo so that would be temporary. And hard water tends to interact with shampoos and conditioners. And if we over-use silicone products, that can have unpleasant cosmetic effects.
DeleteThanks for the reply. Darn-- I'm just running out of ideas about what to do with the 21 gpg water hardness in my municipality. Hard water shampoo doesn't really seem to be doing the trick: my hair never seems to lose that grippiness.
DeleteLishan - have you tried using distilled water with your hard water shampoo? Distilled water contains no minerals, so you would not only be using a shampoo that removes minerals (or at least reduces their interaction with hair), but there would be no minerals in the water to confound the process.
DeleteThis is a bit extreme.. but you could use a shower cap to protect it and then wash it separately in bottled water.
DeleteI hadn't tried either of these, actually! I'll give it a try. :)
DeleteLishen, if you find that you're just miserable with your water and the distilled water works for you, you could consider buying a distiller. I have one and I always do an initial rinse with it and then the final rinse. It was very expensive to buy the gallon jugs and not worth it to travel to friends or neighbors that have decent water. I use about a gallon a day and have had my distiller for about two years. I run it pretty much every single day and have not had an issue. The one I have is from Amazon and is called Pure Water Distiller by Selani. I cannot do the entire wash, rinse, condition and rinse with it because it's just too stinking cold, so I do battle with green in my hair. But, I have also found that Redken Pre Art is a miracle worker at removing the minerals from my hair. I am not sure if it is just my particular water or if it works like this for everyone with a head full of minerals, but it's worth a shot if you're all out of options.
DeleteIf you have a TDS (total dissolved solids, for any laypeople reading this) of 500, you must have high alkalinity water. I don't mean alkaline as in high pH. Alkalinity is the kind of mineral hardness that isn't removed by most water softeners. Alkalinity minerals in water don't have the right ionic charge for water softeners. Water softeners are made to remove positively charged ions, but they can't remove negatively charged "alkalinity ions."
DeleteMy regional water has high alkalinity and high hardness. They treat it with "lime softening" at the municipal treatment plant to precipitate out a lot of the minerals that cause the TDS to be high, and that makes the water softer - and easy to soften with a salt softener. Lucky me, right?
Mineral enhancers on reverse osmosis systems add minerals back to the water, and it's bringing the pH back up too. If your water's pH is too low, you run the risk not only of corroding the pipes, but also of leaching lead out of them.
So you still have more minerals in your reverse osmosis-treated water than in the distilled water. If you could use the non-re-mineralized RO water on your hair, it would probably be fine.
This is not a problem that can be corrected with most home treatment systems, otherwise. You can use a countertop distillation pitcher (they look like big coffee pots) and that will take out the minerals and give you water with an ideal pH. But you can only distill 1 to 3 gallons at a time, and it does use electricity to heat the water and a fan to cool it in order to distill. You then have to clean out of the distiller's heating compartment occasionally, which is surprisingly satisfying.
I get it - most people can worry about products and hairstyles and something as simple as your water is sabotaging your hair.
Sorry this is a problem - and I hope that helped shed some light on the situation. Best wishes, W
Hello,
ReplyDeleteI’ve been following your blog for some time, and it has been extremely helpful to me. I wanted to see if I could get your insight on a hair challenge that I am dealing with.
For the last few months, I have been coloring my lower-porosity, light-medium ash brown hair with a semi-permanent red direct deposit dye called "Jazzing." From my understanding, Jazzing is basically pigment suspended in a hair gloss. In the last month, I’ve made the decision to go back to my natural color. I have tried several methods to fade the dye including repeatedly washing it with strong sulfate shampoos, such as Head and Shoulders; letting the shampoo sit on my hair with baking soda for 10 minutes; soaking my hair in water containing bath salts; and using hot oil treatments. The oil has helped slightly, but I have not achieved the results I am looking for. My hair is still quite red and I can still feel the texture of the gloss on my strands. I have tried to color over it with a semi-permanent and with a permanent oxidative dye. However, these dyes did not cut through the Jazzing. (I did strand tests when using the oxidative dyes so my hair is still in decent condition overall).I wanted to ask if you know of any ideas that I could use to remove the Jazzing, or effectively conceal it.
Thank you.
Hello AMD,
DeleteThat is supposed to be a temporary color that fades gradually in about 12 washings. That said, if you used it with high heat, it will be more permanent.
Because you have colored over it, this gets tricky. I'm not sure if a product like Color Oops or L'Oreal Colorist Secrets Color Remover will work on a gloss or not. You would want to do a test-strand for sure in case you got an off-color from those.
Continued coloring will probably just make matters worse. The product (Jazzing, and glosses in general) forms a lasting film over your hair and until that is gone, the color will stay. You may just have to wait it out because it can't be removed without very nasty solvents.
For color-correcting to tone down the red shade:
You might try Ardell Red-Gold Corrector, which is a little bottle of purple additive you put in your shampoo or conditioner - it might cool down the red shade a little. To try to cancel out the red shade, you might use green (as in, a couple drops of green food color added to your rinse-out conditioner).
I hope that helps!
Thank you for your response. I had been a bit reluctant to try a color remover, but it sounds like it is worth a shot. I will be sure to do a test strand. I had not considered color correcting my hair before since the red is pretty cool-toned, but the idea of using green food coloring is brilliant! I will have to get some the next time I go to the grocery store :) Do you think its okay to use it in a leave-in conditioner, or should I just stick to the rinse out? Thanks again for your help.
ReplyDeleteI know using a color for color-correction works with blue and purple (for toning down brassiness) - but it has to be much lighter than you would use in a shampoo. When you have a bit of time, mix up 2 or 3 different mini-batches with increasing darkness (green color in leave-in conditioner) and test them on some strands to see how much color you need and how much is too much.
DeleteI just wanted to add my experience here because maybe it can help someone else. I have very high ph well water that is also loaded with minerals. For seven years I have been struggling with trying to keep the green out of my blonde hair. Malibu shampoo, conditioner and packets have been the only things that have made a difference but still my hair has always kept a tint of green and has never felt as soft as it did before moving here. I am constantly trying new things and searching for solutions but nothing has worked very well. A few weeks ago I was on a hairdressing blog and someone mentioned Redken Pre Art as a very strong chelator but also very gentle. I had never heard of it before so I started searching, found it and immediately ordered it from Amazon. I was so excited that I had it over-nighted and the next day it was in my hair. I saturated my hair with it, put a cap on and left it for a half hour. I then spritzed with water, put a little Malibu shampoo and as it bubbled up, it was green! The green just started sliding out of my hair. I kept spritzing and squeezing the green out until it was entirely gone. My hair feels like hair, it is blonde, no green at all and feels so healthy that I'm amazed no one has mentioned this product before. It is honestly like a miracle product to me. I sent a question about the Redken as well as the ingredient list to thebeautybrains.com and they said that the ingredient that is working so well is Trisodium Hedta. I have tried to find shampoos and conditioners that have this but they are never high on the ingredient list. I would love to find something to use everyday to keep the minerals out, but so far I am using the Redken every week to keep my hair from turning. Hopefully this info can help someone else who is having this issue.
ReplyDeleteHello Kat Jo,
DeleteThanks for sharing your experience! EDTA (Trisodium, Tetrasodium and Disodium) are all mineral chelators. They're molecules that have a place to grab minerals. Hemoglobin is a familiar chelator - it grabs oxygen and carries it to where it needs to go. If somebody has lead poisoning, they are treated with chelating medications to try to remove the lead from their bodies (it's very difficult to do without causing other problems).
You're right, most products you can buy at drugstores do not contain high amounts of EDTA. That's because mostly it is used to stabilize a product and as a co-preservative. It's pretty cheap, that's not the problem, but to make a product with a lot of it, you have to change the formula around. So you have a "treatment" like this rather than a conditioner or shampoo.
EDTA can be purchased as a powder and added to shampoo if you feel adventurous. I wouldn't go higher than 1% for safety reasons (though I have not seen the ingredients for Redken Pre Art). This is not something you want draining into a place where frogs and birds live - it's not benign. 1% is a weight measurement. 1 gram EDTA per 100 grams of other liquid.
Citric acid is also a chelator. It is less gentle because it is acidic. But if you haven't tried citric acid rinses, you might. They work best when left on for a few minutes with heat before rinsing.
Sounds like you have copper in your well water - copper will turn hair green. And copper IS chelated by things like EDTA and citric acid. If your water were low pH, then you might worry about copper plumbing that was damaged and therefore adding copper to the water. But because your water is alkaline (high pH) that doesn't necessarily apply.
Redken also makes a shampoo called Hair Cleansing Cream which is typically recommended for daily/routine home maintenance for hair that needs consistent attention to hard water amd mineral build up.
DeleteThe Redken formula has Trisodium HEDTA listed as the second ingredient after water, so I'm guessing it's a pretty high amount. I would love to make a cheap version of this that I can use on a daily basis, do you think it is safe for hair everyday?
ReplyDeleteWhen you say that it is not benign, should I worry about the safety of it on my 13 year old because I have done her hair twice now.
I have tried citric acid, I think some of the versions of Malibu treatments contain a lot of citric acid but nothing works as well as the Redken. I have used lemon juice, vinegar, even tried aspirin but I think this specific ingredient just works like a miracle in my hair. Do you feel that the Trisodium HEDTA is equally as effective a chelator as the others or is it somehow different?
I've heard that the higher the ph of the water, the more likely the minerals are to cling to the hair, is that also accurate?
My feeling is that if this is your miracle product - keep using it. Use it again when your hair shows signs of needing it. I prefer to turn to making my own products only when desperation or discontinuation of something I love requires it. If a good product exists - cherish that, pay the price if you can afford it and economize elsewhere. Watch for sales and take advantage of them. If this product is sold locally - there can be some really good deals if you keep an eye on sales and store coupons.
DeleteWith Disodium or Tetrasodium EDTA I would start with 1-2% for occasional use. Depending on their other ingredients, there may not be much more than 2% in the product - ingredient lists are given in relative amounts. EDTA is low toxicity used topically in normal amounts, but when we're making products, we have lots of things to consider. http://www.ncbi.nlm.nih.gov/pubmed/12396676 EDTA it should not be breathed or ingested (obviously)! The higher the concentration, the more likely it is to become an eye irritant or skin irritant, so that is one problem to avoid by keeping the "dose" as low as you can while still getting the product to work. Your 13 year old is probably fine - but take care to keep it out of her eyes and if any skin irritation develops - stop using it.
Out in the world once rinsed down the drain, EDTA does not degrade readily. So just like in your hair, it can affect mobility of metals in the water. It's not entirely known what the end result of that is for all living things that rely on that water, but better to use as little as needed.
Malibu treatments won't contain enough citric acid to do any good, hard-water-wise. When you use citric acid with heat on your hair, it will smell metallic when you rinse it. Not all that is due to metals - but it makes it feel like it's really working. If it's not working for you, though - then it's not working. Vinegar doesn't work on my hard water problems either. And my hair hates it.
Yes, the higher the pH of the water, the more calcium (and magnesium) will bond to your hair. And probably copper too - no reason why not.
You're unlikely to be able to buy Trisodium HEDTA as a private citizen (not a chemical company). It has some effectiveness at high pH and good solubility - EDTA doesn't always want to dissolve. You'll have readier access to the other 2 common forms.
Ok, thank you so much, I will stick with the Redken for as long as they make it. I definitely do not want to mess around with chemicals. I might try the citric acid with heat, I have never used heat with it so that could make a difference. I appreciate your help so much!
DeleteThank you for the replies, I still have one to find. It is amazing how stress, which I have had a lot of, and I do overthink, and have had counselling for it, but this is so time consuming, but I have not choice to keep finding out. I heard that jojoba oil is good. the other day i mixed a few drops of peppermint with tea tree and a carrier, and it helped a little, but it is back being itchy today. it is like a nightmare. i dont remember what i wrote exactly, but at the crown there seems to be a bump that has coarse dandruffy granules, flakes, and it is bad sometimes, and not others. in that area, there is a lot of hair missing and some new hair starting. i never had dandruff, or the flakes, i am upset as can be, but i will not stop , and i appreciate all help, and it can be passed on from me, too, to yoou all, that wish to read it. i am worried that the top front all the way back to that crown will thin, i hope there is a thickner, natural, thanks, mary
DeleteAt any time you can have a brand new reaction to a product or product combination. Stress can set your skin up for that! As before, see a doctor if it's not getting under control on your own. Hydrocortisone is good for itching and inflammation and often reduces flaking after a few applications - especially good for localized patches. Salicylic acid helps reduce flaking and is also anti-inflammatory, but it can be irritating to some people's skin.
DeleteI know people who've had their faces swell up from hair dye reactions, eyes swell shut, lymph glands swell in the neck, rashes... Bad reactions are frustrating and upsetting and uncomfortable. The sooner you get the symptoms under control, the better you'll be able to figure out how to avoid them in the future. Good luck!
Just thought I'd chime in really quick -
Deletehttp://www.curlgirlhair.com/clean-curls-sulfate-free-moisturizing-shampoo.html
That product has disodium EDTA as the second ingredient too! I bought at Marshall's today, and an plagued with hard water too, so I'll report back after I use it.
hi, i have been having a very mysterious, horrendous problem with my scalp for three or four months. it started out when i used head and shoulders when removing hair color, and it caused a reaction, then it turned into scalp sores, and horrendous itching, flakes, so many. now it is some scalp bumps that if bumped, they have hard, big flakes in them, and if i even slightly itch my scalp, it is like the skin is thin on top, and out pours this very fine powder, it is alarming. some hair has broken. i am a hairdresser, but i have never had dandruff, or any scalp problems, ever, and i did go to two salons for my own hair, but dont know what this is, do you have any ideas? it is so annoying, and worse, hard to take my focus off of, because i have no answer. thank you, mw
ReplyDeleteI replied to your other post as well. That sounds like irritated/inflamed skin as a reaction to the hair dye, the Head & Shoulders (or some combination of the two). Inflamed skin will often start to over-produce skin cells. If you scratch - you get a little rain of powdery flakes. Eyebrows do that too sometimes.
DeleteYour reaction sounds like either a sensitivity reaction (to put it mildly!) or an allergy to the dye or the dye + Head & Shoulders, and that may have revealed a skin condition that was lurking but not yet discovered too. The progression from sores (the immediate inflammatory response) to flakes and itching (the residual irritation and inflammation) and bumps is just what you'd expect with contact dermatitis, atopic dermatitis and seborrheic dermatitis, and what they all have in common is environmental triggers and inflammation. The skin becomes more sensitive as a result, infections pop up easily because the skin barrier has been damaged (bacterial and fungal infections - the bumps) and skin cells proliferate more than usual (the flakes).
This is an irritation and inflammation of the scalp that it is not able to recover from using its own resources. You might need topical hydrocortisone (1%, like Cortaid) for the inflammation and itch. Salicylic acid topicals (Scalpicin regular strength or Neutrogena T-Sal) can help with flaking and bumps. If your skin can cope with Salicylic acid - sometimes 1-2 uses will clear up the flakes and bumps very quickly.
But that is all "do it yourself." If you have open sores or open sores with little red halos around them or you are feeling worse than just scalp irritation - best to have that checked by a doctor to see if you need a topical antibacterial treatment.
Try to avoid heat styling and direct sunlight while your scalp heals. If you use hair color again, patch test like your life depended on it! Behind your ear so it's near your scalp. Any sensation at all would be a "no" for that product.
It might be best to use a shampoo other than Head & Shoulders after coloring. I can imagine the pyrithione zinc being a problem for several reasons. One of which is - if your scalp is a little bit irritated from exposure to the high pH of hair color - it might react to the shampoo in a negative way. Under other circumstances it might have been fine. Some people will get irritated scalps from Head & Shoulders. Sensitive scalps can be very contrary.
Good luck!
I agree with the other reply. Sounds like a possible allergic reaction or serious irritation that may have progressed into a bacterial other infection. You can become allergic to a product between one use and the next (hair color always states to do a spot test before every use.. most people don't), and combining chemicals can give unintended reactions. I would see a doctor for starters, it sounds like it's not getting better and you don't want to damage your scalp permanently or lose your hair. Also consider going to a very mild chemical free shampoo and conditioner, I really love Shea Moisture (and they have come a long way with tons of various formulas.. I use the fine hair version and adore it, it's moisturizing and nourishing while being very light/gentle and does not build up at all which is something I'm very prone to). It can be hard to find the various formulas.. I've noticed Ulta, Target, and Sally's have a selection most other stores don't, and they each sell different items. I can only find the full fine hair line at Ulta.. but Target had the shampoo last time I was there.
DeleteHello,
ReplyDeleteI have been reading your blog for a while now and I love it! I just want to ask you for advice.
I moved from the UK to the Netherlands as part of an erasmus course last year. I never changed my hair routine and used the same shampoo and conditioner that I loved. However I realised my hair started going really greasy ,slippery and unmanageable(Even though I was still using the same hair routine ).I tried everything from using less conditioner or no conditioner at all. It wasn't until I got backto the UK that I realised that the type of water in the Netherlands is very soft. I live in a part of the UK where the water is very hard and so probably my hair was not use to the softness of the water. I am moving to the Netherlands again in a couple of months- Is there any hair routine (i.e Type of shampoo ,conditioner)you can advice me to undergo when I am back to using soft water in the Netherlands ?
Kind Regards
Hello Lois,
DeletePeople who grow up with hard water only know "hard water hair." The slightly cheeky response would be to buy mineral water and pour it (or spray it) on your hair so it gets it's usual dose of minerals while you are using soft water. Actually - that can work.
Hard-water-hair people usually end up needing less conditioner when they are using soft water because you don't have the minerals making your hair slightly rigid and more-dry. You might need to look for a lighter-weight conditioner (those for increasing volume are often lighter). Or you may need to dilute conditioner with water when you use it. If your hair isn't feeling dry or rough - then all you need conditioner for is to assist in detangling. If you don't even need conditioner to assist in detangling, skin using a rinse-out conditioner entirely and maybe just apply a little conditioner as a leave-in to very wet hair, add some extra water to dilute it, and then squeeze out the water.
If you are using a pearly-looking or creamy, conditioning shampoo, you may need to switch to one with a clear appearance that has no "slippery" ingredients nor silicone ingredients - not a "conditioning shampoo" unless your hair becomes very tangly or dry.
You might want to use a styling product that creates some friction in your hair like the minerals do when you're at home. A sea salt spray (commercial or homemade with sea salt or epsom salt/magnesium sulphate in distilled water with a tiny bit of conditioner) will add some texture to your hair. Other ingredients that add a little friction include things like shea butter, "dry shampoo," henna, styling gels with xanthan gum or cornstarch, conditioners with Stearamidopropyl Dimethylamine. I hope that helps get you started.
You could also try reverse conditioning... condition first, then shampoo the roots and let it rinse through the ends.
DeleteThank you to the author (and commenters!) for such helpful information. I'm now convinced that my shower water is wreaking havoc on my hair. It used to be soft, shiny, and easy to manage; but now it's easily tangled, frizzy, and dull. The pH of my city water is around 7.7-7.8, but I live in an old house and I've seen rust colored water come out of a tub faucet that we only use occasionally. Also, in the almost 6 years I've been in this house, my hair has turned from straight to very wavy - probably unrelated to the water but still odd!
ReplyDeleteThe Redken Pre Art product sounds fantastic, but I'm finding that it has been discontinued. Is Redken's Hair Cleansing Cream Shampoo the current equivalent?
Hello Mary,
DeleteIt looks like Redken's Hair Cleansing Cream Shampoo is a replacement for Pre-Art, I'm not sure it's an exact equivalent. The ingredient Pentasodium Pentetate is the chelating or mineral binding ingredient in this product.
Fascinating (and overwhelming) information on your blog! I have struggled with hair dryness and stiffness for years. Most recently I've tried co-washing and was very happy with the results at first (lots of curl I didn't know existed appeared). I'm having mixed results as the dryness and stiffness has returned.
ReplyDeleteIn the middle of all of this self-experimentation, my 8th grade daughter chose hair strength for her science project. She got 20 hairs each from me, her science teacher, a friend, and herself, and tested the tensile strength of 3 hairs from each person. She then washed 3 hairs from each person in 3 different shampoos (not the same shampoos--3 hairs for each shampoo--1 moisturizing, 1 keratin, 1 panthenol, and 1 control set washed only in water). Then she measured the hair strength of each of those hairs at the end. She learned a lot the hard way, discovered many variables along the way, and in the end found hair strength wasn't significantly changed in any sample. That was kind of discouraging to her, although she is learning that failures are still a valuable part of learning--whether about the need to refine methods or opening the door to new things to consider. E.g. perhaps matching conditioners might have needed to be used with the shampoos to make the promised difference in strength, if they are effective at all. And testing more people's hair and more hairs from each person would have been better. However, we (it took 4 hands) are both so tired of trying to manage and weigh hair strength the long, slow, hard way I don't think she’ll take this experiment further any time soon! It does give us huge respect for all the science you do with hair, although I imagine you have better technology than ours (we taped hair to a pencil and styrofoam cup, added pennies 1 by 1, and measured the cup after the hair broke. Times 48 hairs. Yep, we're a little sick of hair right now, lol). She was going to compare all the hairs under the microscope, but ran out of time, since managing, washing, and weighing the hairs took longer than expected, and strength was her focus.
One discovery was that her hair and mine were almost double the strength of her teacher's hair and 3-4 times as strong as her friend's. We aren't sure the benefits of wide, strong strands, though, since both of our hair is really dry and dull, and mine is often stiff. In the past, protein shampoos have dried my hair. In this experiment, it didn't seem to make any difference in strength, although there are obviously other variables we didn't look at.
Which brings me to your post and wondering if our hair woes are related to hard water. Our city water leaves white buildup on our appliances. Soaking those areas with vinegar-saturated rags helps. I recently sprayed vinegar-diluted-with-water on my hair before shampooing, and it left it soft. I was hesitant to do it often, because of conflicting reports, including people who said it softened hair for a while and then left it completely stripped.
To make it more confusing to me, years ago a stylist recommended that I put baking soda in my shampoo every time I washed. That also softened my hair for a long while, but at some point didn't. And now, I wonder why it worked at all, since baking soda would do the opposite of vinegar, right? And a friend recently told me her aunt completely wrecked her hair by using baking soda on it regularly. Yikes-so many factors at play. The list of chelating shampoos is overwhelming, too, since I mixed results with different products and can't figure out which parts cause what problems, except for protein, which seems to make my hair consistently dryer. I'm a little scared I'll end up with several partially used bottles that make my hair feel worse before I find one that will work (I've already done that with shampoos, conditioners, and leave-in products.) Just read about your hair analysis yesterday, and that sounds like a great next step for me.
What a great project! Not only because it is something easy to relate to, but also because your daughter got to experience the frequently laborious, occasionally tedious, trial-and-error of actually doing science. Let me tell you about the month I spent trying to work out a problem I was having with one of my reagents interacting with the soils I work with... Well, maybe not - but it was a whole month of trial and error and hypothesizing and teasing out a solution. Good for her! I hope she's very proud of her persistence. I'm pleased to hear about it. :)
DeleteIt does sound like you have hard water. Depending on the actual chemistry of that hard water, you'll get a better result from vinegar or from citric acid or EDTA (the latter in chelating shampoos). If you have high "alkalinity" which you'd find as "total dissolved solids" or TDS - then vinegar rinses may work better than chelating shampoos.
Baking soda doesn't work well for mineral residue - unless you are using it as a physical abrasive to scour out a sink or bathtub.
For coarse hair, protein shampoos are likely to cause a dry feeling because they increase the stiffness of hair. Great for narrower hairs, not so good for coarse hair.
Best wishes! W
Thanks! That's what my husband and I keep telling her. It may take a little time and distance for her to believe us that she should be proud of her hard work. I know she's learned more than she knows right now. I'll definitely tell her about your month trialing and erroring it for your profession.
DeleteAnd it definitely sounds like doing some kind of hard water treatment for my hair regularly might be a good thing.
I am so glad you bought this up, my water makes my hair so dry and I got a free water testing kit from http://www.fabfreesamples.co.uk/free-hard-water-testing-strip/ and tested it and the results were off the charts, my husband has to install some compositor that cleans it.
ReplyDeleteHi W,
ReplyDeleteThank you so much for this article! I've lived in Seattle and NYC my whole life and recently moved to SF. I went from having soft, shiny hair to brittle, dry, frizzy and unmanageable hair. I bought waterstick's shower filter but even that didn't help much. However when I go to Seattle my hair is soft! Would you suggest me trying Redken's cleansing cream? I've tried almost everything. I tried Ion's hard water shampoo before I got the waterstick's and it didn't do much. I even tried rinsing my hair with spring water but didn't think of distilled. I'm so desperate for hair that doesn't feel like straw! Thank you so much.
Hello AK,
DeleteI'm sorry this reply is so late. Busy, busy, busy! Redken Cleansing Cream has an interesting ingredient, Pentasodium Pentetate. It's like EDTA. It's worth a try - it looks like a very nice shampoo, based on the ingredients.
Citric acid (rinses) and EDTA and Pentasodium Pentetate all work on hard water minerals with a positive (+) charge. There are hard water minerals with a negative (-) charge that the Watersticks cannot remove, and citric acid, EDTA and Pentasodium Pentetate don't work as well on.
So if those fail you - you might still do well with vinegar rinses - which work in a different way.
And if you're still getting hair that feels very dry even with the Waterstick softener and you're recharging it with salt as often as you need to - I wonder if that's the case. If you can check your water quality report online, see if they mention "alkalinity." If it is close to or over 100, then citric acid, EDTA and Pentasodium Pentetate won't work very well. But vinegar might.
With the low humidity and wind - you probably want to have "film-forming humectants" in every product you can manage to have them in for hydration. Here's what I'm talking about: http://science-yhairblog.blogspot.com/2014/07/film-forming-humectants-what-they-are.html
Good luck! -W
Would any of these hard water shampoos that you mentioned also work to remove product (shea butter, oils) build up? I've recently gone silicone and sulfate free and wondering if there is one shampoo I can use for both issues so as to limit the amount of shampooing I need to do.
ReplyDeleteHello angelaoversees,
DeleteThe shampoos in this list should be good at removing shea butter and oils. The Malibu demineralizer treatments do contain a detergent, but I'm not sure how effective it would be at removing butter build-up. They should work, but a shampoo might be more economical.
I've read that Aveda Shampure is a chelating shampoo, but they don't advertise it as such. It contain sodium gluconate. Would you consider it a chelating shampoo? Here is the latest ingredient list from their website:
ReplyDeleteINGREDIENTS
Ingredients: Aqueous (Water\Aqua\Eau) Extracts\Extraits Aqueux:Lavandula Angustifolia (Lavender) Extract, Aloe Barbadensis Leaf Juice, Rosmarinus Officinalis (Rosemary) Leaf Extract , Ammonium Lauryl Sulfate , Disodium Laureth Sulfosuccinate , Lauramidopropyl Betaine , PEG-6 Cocamide , Dimethicone PEG-7 Isostearate , Babassuamidopropyl Betaine , Hydroxypropyltrimonium Hydrolyzed Wheat Protein , Hydrolyzed Brazil Nut Protein , Wheat Amino Acids , Persea Gratissima (Avocado) Oil , Glycerin , Tocopheryl Acetate , Ascorbyl Palmitate , Guar Hydroxypropyltrimonium Chloride , PEG-30 Castor Oil , Polyquaternium-7 , Sodium Sulfate , Fragrance (Parfum) , Geraniol , Linalool , Citronellol , Limonene , Sodium Chloride , Polysorbate 80 , Sodium Gluconate , Maltodextrin , Potassium Sorbate , Sodium Benzoate , Phenoxyethanol , Annatto (CI 75120)
Hello Rusty22,
DeleteYes - that could be considered a chelating shampoo as long as the water isn't extremely hard. The harder the water, the more Sodium gluconate is required to work well.
Thank you!
ReplyDeleteHi... I'm based in India. Last two years of moving into hard water area has significantly damaged my hair. From straight and silky they have become extremely frizzy and wavy and dull. I can considerable raused cuticles. Been using distilled water for hair wash for 6 months. It has stopped further damage but unable to undo the damaged hair. Recently used aubrey organics swimmers shampoo and conditioner. It's extremely drying and not working. Can you pl help
ReplyDeleteHello Aastha,
DeleteI'm afraid I have used that same shampoo. I did not like it either. If you have not tried a vinegar rinse or a citric acid rinse (see DIY homemade rinses above) - you might try that. Distilled water does not add any minerals to your hair, but it also does not help remove them. Vinegar or citric acid can help remove them from your hair.
Because hard water tends to make hair less flexible, you might need to use oil in your hair for several hours before washing. Coconut oil is good - but sometimes it can also make hair dry and dull. Sunflower oil and avocado oil are good if coconut oil does not work for you. Good luck! -W
Hi thanks for your response. Recently I've also tried one in salon treatment of malibu c and also home rinses with citric acid/acv. Unfortunately nothing seems to be working. I read that the tds level in our city is well above 7000ppm. I'm so disappointed and hoping that the damage is not irreversible. The waves and kinks in certain sections of the head also look stubborn. What can I be doing.. thanks
DeleteAsatha,
DeleteMinerals accumulate on the surface of the hair and inside the hair. The salon Malibu treatment uses heat and a long treatment time to try to remove minerals from the inside of the hair also. With high TDS, that is a sort of "hardness" which is more difficult to remove from hair. Vinegar tends to work better. I know some people who use half vinegar, half water for water like that. Which can be drying because it is more concentrated, so you must be careful.
If possible, keep using distilled water to wash your hair. Do not use soap bars on your hair - that makes the mineral residue worse. Avoid high-heat styling, highlighting and permanent coloring if you can. Those treatments make hair more porous - and porous hair takes up more minerals from the water. Use oil treatments on your hair for several hours before washing to keep it healthy and flexible - the healthier your hair is, the less porous it will be a the fewer minerals it will take up.
Best wishes - W
Thanks so much... any experience if the waviness texture caused can be reversed to original straight hair over a period of time? Or is it a permanent damage to the follicle structure? I've already started to use oil and vinegar as you suggested. Thanks a ton.
DeleteHi, I posted on here back in April 2016 about my terrible water and what I was doing to manage my color treated hair. No matter what I did, it was never nice, soft, shiny or healthy like it was before we moved here. Not only did our water have extremely high copper, a very high ph, but it also has colloidal clay. If you don't know that that is, you're lucky. It's very fine clay particles that suspended in the water and are usually too small for a filter. This was all winding up in my hair, turning it green and making it feel so heavy and dry. We've been in this house for ten years now so I've figured out quite a bit. Last summer we took the plunge and spent $6000 on a whole house reverse osmosis system. Outrageous I know, but if you can't drink your water, cook with it or bath in it without issues then what good
ReplyDeleteIs it? Anyhow, the system is not perfect. I have issues basically every month, when it rains, I need to adjust things, pressure changes, adjust things, drought, adjust. You get the idea. So it's nice when it's good but a few days of not testing, my hair turns heavy. The chemicals to treat the colloidal clay are unbelievably expensive, I tend to neglect that and it shows in my hair. So with all that said, here's what info I have that might actually be helpful for those of you who do not want to spend $6000. I mentioned before a redken product that is amazing, but of course it was discontinued so that helps no one. With this insane rain we've had in NY lately, the clay is really unbearable. I went into Sally's for swimmers shampoo for my daughter and discovered an amazing shampoo and conditioner. I'm pretty sure it's new, I've never seen it there before but it's in with the ion brand products. A teal and white set of bottles that say -hard water shampoo and conditioner- they're not expensive though I don't recall how much. The first time I used it I washed my hair twice to really clarify it. I only use a tiny bit of conditioner and do the opposite of what were all used to, I rub it on my scalp and I'll explain in a second. Rinse like mad and then plop a towel on it. I found another product called d-chlorine-8 online just by chance. It's $20 a bottle but It's a leave in conditioner that prevents the minerals and junk from sticking. I was skeptical but after using it's once, my hair was soft, no crunchy or heavy feeling, and shiny for the first time in forever. I'm not sure if it's just the shampoo and conditioner or a mix of that and the spray but for me, this mix has made a world of difference. The spray can be found online, they have a website. Just let me know
If anyone can't find it. I would only purchase
It from their site directly if you're going to, I've had a lot of fake products coming from other sites lately. If you have this horrible water, definitely give the ion shampoo and conditioner a try, it's inexpensive enough to take a chance.
Thank you for the update! Sorry the water system is so frustrating. Clay is indeed very difficult to filter from water. I added the DChlorin8 to the list. It should at least prevent minerals from interacting too much with hair while it's in the hair.
DeleteHey! I just found this blog and it's super fascinating and I am definitely going to be reading a lot of this. This article in particular caught my eye because I've had a query about my hair that no one has been able to answer, but I was wondering if you might know why.
ReplyDeleteWhen I travel to other areas of my state, there have been a couple areas where the water has made my hair spectacular, the type of hair I want all the time. I also am able to use a fraction of the shampoo and conditioner that I normally do when I visit these places. They've been in places where there is well water (or what is basically well water), and it's iron heavy. The sort of water that is unpleasant to drink or look at! I have fine, low porosity curls as well. Everyone else I've talked to says that this sort of water ruins their hair! If you have any idea why that might be the case (and perhaps how I can replicate it without having to travel to areas with water like this) I'd love to know!
Hello Mikayla,
DeleteYou seem to be describing very hard water versus less hard water. Less hard water requires less shampoo to make lather.
Depending on which state you're talking about - there can be other things like pH (higher pH water in dry/desert areas tends to taste bad and make very little lather with shampoo).
Without knowing the hardness or pH of the water, I can't make a very accurate guess. Harder water tends to add "grip" to fine, curly hair so the curls don't slide out. But hard water usually requires more shampoo to lather. So it may be a subtle difference in water chemistry. I would have to know the locations to make an accurate guess. And you might be able to use the link to the map above (it's bigger) to see what the difference is. -W
Hi! Is Briogeo Be Gentle Be Kind Clarifying Shampoo a good option for removing hard water buildup? How often do people normally need to remove hard water if they live in areas with moderately high hard water - once a month, once a week?
ReplyDeleteHello Aileen, My guess is that is probably not a good shampoo for removing minerals from hair. It does contain citric acid, but very probably not enough. If you have moderately hard water - every 3-4 weeks is probably enough for a chelating (mineral removing) treatment. If your hair is the sort that really shows hard water residue, you might use it more often, or do a distilled water wash somewhere in between. Best - W
DeleteDevacurl buildup buster uses micellar water and claims it removes hard water accumulation. Does this sound legit? Here are the ingredients.
ReplyDeleteINGREDIENTS:
Water (Aqua, Eau), Lactamide MEA, Glycerin, Crambe Abyssinica Seed Oil, Limonium Gerberi Extract, Cynara Scolymus (Artichoke) Leaf Extract, Hydrolyzed Jojoba Esters, Hydrolyzed Pea Protein, Panthenol, Melissa Officinalis Extract, Humulus Lupulus (Hops) Extract, Cymbopogon Schoenanthus Extract, Chamomilla Recutita (Matricaria) Extract, Rosmarinus Officinalis (Rosemary) Extract, Achillea Millefolium Extract, Caprylic/Capric Triglyceride, Hydroxypropyl Guar Hydroxyproplytrimonium Chloride, Hydroxyethylcellulose, Cocamidopropyl PG-Dimonium Chloride Phosphate, Cetrimonium Chloride, PEG-40 Hydrogenated Castor Oil, Polyacrylate-1 Crosspolymer, Polyquaternium-10, Polyquaternium-11, PPG-26-Buteth-26, Propanediol, Disodium EDTA, Citric Acid, Phenoxyethanol, Fragrance (Parfum).
Hello Chelsea,
DeleteIt's legit in that is will absolutely form "micelles" which are one of the ways that detergents (and soaps) work to remove oil, dust, dirt and other residues. It should be able to remove excess oils and any other "stuff" in hair. I see 3 ingredients that can cause a feeling of build-up for some people - Guar Hydroxypropyltrimonium Chloride and Polyquaternium-10 and Polyquaternium-11.
Is there enough EDTA to remove mineral residue? Possibly.
Hello there!
ReplyDeleteFirst of all I wanted to say thank you for the amazing blog and all the super helpful amount of information here! I have started my curly hair (curly girl method) journey about a month and a half ago and I have to say that this so far is the place where I have found the most amount of helpful information that is helping me understand sooooo much about my hair and how it reacts to different products (it seems I am an odd one and all the supposed great products that seem to work for everyone else do not work for me) :(
I'm afraid my question is about EDTA (and not minerals and hard water). Since this is a place where EDTA is mentioned quite often, I thought I would ask my question in this thread. I hope that's ok.
I wanted to know your thoughts on Tetrasodium EDTA (is that the same as Disodium EDTA or are they different things?). Is that a toxic ingredient? I have read in some places that it is toxic and even cancirogenic. I assume it depends on the amounts used but I wouldn't want to put anything on my hair that would be detrimental to its health on the long run. Also, would it be drying to hair, pull out moisture or mess with the natural proteins/oils in the hair?
I'm asking because I see it as one of the (last) ingredients one some gels like the ECO styler Curl & Wave gel (Here's a list of the ingredients: Water, Carbomer, Hydrolyzed Wheat Protein, Glycerin, Amino-2-Methyl-1-Propanol, Sodium Hydroxymethylglycinate, Polysorbate 20, Tetrasodium EDTA, Fragance, Red#33) and I was wondering if it should be avoided.
I was thinking of trying that gel but since it's quite cheap I'm worried some of the ingredients might be harmful in the long run.
Thanks in advance for the help!! :)
Disodium and Tetrasodium EDTA are very similar and used for the same purpose in cosmetics - to stabilize the product (which helps it have a longer shelf life), to prevent minerals from interfering with the product's action and in some cases, to help with hard water minerals. These ingredients are not drying to hair and are used in very small amounts - usually less than 1%.
DeleteEDTA should not be eaten, that can be weakly toxic or cause birth defects. Testing done with the amounts used in cosmetics have found it far below the amounts which can cause reproductive or developmental toxicity. The FDA report mentions it has not been found to be carcinogenic. Here is a link to an abstract/summary for that report. http://journals.sagepub.com/doi/abs/10.1080/10915810290096522?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub%3Dpubmed&
So for *you* it is unlikely to be a problem.
As an environmental toxin - once you wash it down the drain - EDTA can increase the content of heavy metals in surface waters - and for the same reason it's a good remover of minerals in hair products. Heavy metals in waters is not good news for wildlife, nor for water treatment. Most of that EDTA comes from detergents used for cleaning - or in paper milling and other industrial applications.
In the case of the product you mentioned - there is no reason to avoid it for your hair's sake. EDTA in hair gels like this is a product-stabilizer.
I just wanted to thank you for your blog. I have learned more about my hair in two posts than I have in a lifetime. I'm off to explore more. 😊
ReplyDeleteHello J.T., I'm so glad you're finding the blog helpful! Best wishes - Wendy
DeleteHello. you've mention in the post regarding hair being ok with acidic rinse. What does that mean? what should i look for or take care of? dryness? breakage?
ReplyDeleteI have the waterstick filters (it was a huge research until i found them) but i think that before regenerating it, water is some how hard... in the long term, that will affect also, right? So maybe i have to do a special shampoo every a few months. What do you think?
Also i was wondering if the K-Pak Clarifying Shampoo is removing hard water residue as product build up.
Regarding Ancient Sunrise Rainwash mineral treatment (ingred.: Citric Acid, Ascorbic Acid, Xanthan Gum) do you have an opinion on this?
TIA!
Natalia
Hello Natalia, If hair is okay with an acidic rinse, it might seem softer (but not *too much softer*) after using one, feel a little slippery when the rinse is on the hair, and won't feel rough or dry or stiff afterwards when the hair has dried. A bad reaction would be overly soft, overly limp, dry, brittle, rough or dull-feeling hair. The limpness can come from dehydration from using a too-strong acidic rinse.
DeleteWith a Watersticks filter, you need to drain it and add salt water weekly (usually) and also backwash twice per year, and replace the filter yearly. It will work less effectively if you don't do that regular maintenance. I need to backwash mine, it has become difficult to recharge the last couple weeks. --- I don't think Joico K-Pack shampoo will help much with mineral residue Ion Hard Water Shampoo or Malibu Hard Water Shampoo would be good choices. I have used Ancient Sunrise Rainwash mineral treatment and I find it much too concentrated at the dilution they recommend. I would add more water. The same applies to Malibu Hard Water Remedy and Ion Crystal treatment, (similar to Malibu) they are very concentrated and can be diluted with water more than is recommended. Some people with very healthy, strong hair can use them, others will experience dry, stiff or flat hair. --- Everybody's hair has a different tolerance for acids which will depend on how wide your hairs are, how healthy your hair is (cuticle damage, chemical processing, heat styling, sun damage, swimming). My opinion (or my recommendation) is always to test a strand of hair first before using anything new all over your hair. Someplace you can see and handle easily. And if in doubt, go with a conservative pH level. I use homemade citric acid rinses for hard water. 2 Ingredients, distilled water and citric acid. Cheap, effective, and I control the pH. The "recipe" is listed above. You can use vitamin C (ascorbic acid) in place of citric acid, or replace half with vitamin C. Best wishes - W
How can i know if my hair is ok (or not) with acidic treatments? What should i look for?
ReplyDeleteTIA
Hello Buchuris. It is best to start with dilute acidic mixtures. The "recipes" I listed above for citric acid and vinegar are strong enough to work, but not overly strong. Healthy hair is less likely to be damaged by those concentrations, with fragile or damaged hair one needs to proceed with caution. If in doubt, either save some shed hairs, tape or tie them together and try the acidic rinse on them, or try it on a small section of hair before using it all over.
DeleteIf your hair does well well with an acidic treatment, you might notice your hair feels softer or more flexible. It may have more shine or look brighter. It may feel lighter and move better. [Hair that feels too fluffy and light-weight could mean the treatment was too strong or left on too long]. If your hair is wavy or curly, the curl pattern may gain definition or bounce.
If your hair does not do well with acidic treatments, you might see very flat hair, very fluffy and overly light-weight hair. It may look dull or feel dry and rough or tangly. Good luck! -W
Hi,
ReplyDeleteThank you for this information. I'm hoping you can offer some guidance on how hard my water is and how best to treat my hair. Our most recent water analysis for my city was in 2003 and the results are here: http://www.fernie.ca/assets/Residents/docs/Water%20Quality%20Analysis.pdf#search="Water%20ph" In summary, ph is 8.26, hardness is 132, alkalinity is 119. There's many other measurements in the table, I'm not sure what's important and how to gauge if they are low or high.
My kettle has calcium build up. I have been one of those people who never can grow hair past their shoulders without it breaking. I've been a hair straightener for a decade and only recently embraced the curly girl method last month in an effort to ditch heat tools and get my hair healthy. I'm still trying to figure out my hair condition. It's about a 2b/c curl, fine but lots of it, breaking, split ends, and protein I'm still trying to figure out. It has no elasticity so I'm trying to moisturize. It feels rough and dry and I was thinking it was overloaded with protein but I've been protein free for a month with no change and after reading this I think it could be related to my water.
Based on the data, how should I proceed? What product or DIY treatment would you recommend? Any products that are CGM approved? Thank you kindly!
Hello Carley, this response is much delayed. You definitely have hard water, I need to put a table for that in the post! Your alkalinity is high too, which is another measure of minerals in water. Your pH is a bit high - enough to make even more mineral deposits on your hair than if you water were pH 7.
DeleteI don't think the Curly Girl book mentions any acidic treatments - only baking soda, which is not for hard water. The citric acid and vinegar rinses can work very well for mineral residue if you use them "right." Meaning - leave it on for a few minutes with some heat. I think that would put them in the realm of "CG acceptable" treatments because there is no detergent and no silicone. The shampoos listed above are (mostly) sulfate-free, but some can be pretty strong-cleaning, so if you avoid shampoos, that will be something to consider.
You can actually do a "diagnostic" test by doing an entire wash-day with distilled water, which is free of minerals. It's a hassle, but manageable if you're only doing it once. If your hair is less-dry and feels much better doing that - then you'll know hard water is a problem. Good luck!
The Malibu products in the packets are pretty strong - some people love them, others (like me) find them irritating to skin and drying to hair.
Hi, I just wrote a long post but it disappeared so here it goes again (shorter version). My city water analysis is in the link below.. in summary, ph is 8.29, hardness of 132 and alkalinity at 119. I'm not sure how to interpret these results and what levels are high and low.
ReplyDeletehttp://www.fernie.ca/assets/Residents/docs/Water%20Quality%20Analysis.pdf#search="Water%20ph"
Based on this data, what would be the best product to combat this? I have found Ion online and can order that and maybe others. I'm in Canada, so product availability can be tricking. Also, what at home DIY treatment would be best based on this data.
My hair is low porosity, 2b/c curl, dry, breaking and I'm one of those people who can't grow hair past their shoulders as it just breaks. I straightened for a decade and now embracing the curly girl method and products to help heal my hair (hopes) Thanks!
Hello,
ReplyDeleteI have low porosity hair, I think the hardness of my water is a problem, I will test it this week-end. If it's a problem, should I use vinegar to rinse, it will be ok for my cuticules ? I do a deep condition, rinse and after I use a leave-in and seal. If I use vinegar I think my cuticule will be close.
What do you think ?
Thank you so much for your blog; I've learned so much from it and frequently link to articles because it's so liberating to have an unbiased, scientific look at hot hair topics and to have them explosives so clearly!
ReplyDeleteI've always grown up with very hard water, evident by the fact limescale forms on practically anything that even sees the water (slight hyperbole, but not a *huge* exaggeration!) Is the scale above referring to Calcium Carbonate in ppm?
A comprehensive scale used for hardness by some water providers in the UK is:
0-50ppm soft
51- 100 moderately soft
101-150 slightly hard
151-150 moderately hard
201-300 hard
300+ very hard
Our water is 314 ppm CaCO3; a neighbouring town is 330. Yet I think the actual quality of the water must vary too, between here and the US, as I read of people having orange and green water coming out of the taps (faucet), colloidal clay as someone mentioned above, and nowhere have I heard of or experienced such anywhere in the UK, except in rare instances when something goes wrong and is quickly put right. It also seems that UK water is fairly consistently around 7.5pH.
My most surprising water experience was in North Wales, where the water is soft (27 ppm) and our cups of tea had no scum on them, nor were there brown stains left inside the cups after drinking the tea! I actually didn't know that was possible! Unfortunately the shower where we were staying was useless for washing my hair properly so I wasn't able to find out how beneficial it could have been for that.
I'm very interested in trying some method of chelation, and perhaps rinsing with 'miracle water' (citric acid + ascorbic acid, diluted) to see if it has any positive effects. I think I have a rich enough conditioner to counteract any dryness chelating might cause, so I'm quite excited to give it a go!
Hello Musical Lottie - I like your "borderline hyperbole, but not really" - I've seen sinks like that. The scale I used is provided by the U.S. Geological Survey. I think yours is much more realistic for consumers, though. Especially for people who have very hard water like you. Leaving "Very Hard" at everything over 118 ppm is like saying there's no different between 120 ppm and 330 ppm - and you know that there is! Water so hard - it comes out of the tap in chunks! The UK has extraordinarily complex underlying geology - that's the reason for the crazy distribution of ultra-hard and soft water.
DeleteI think your "miracle water" could be distilled water. Honestly - no minerals so it's soft as can be. Citric acid with a little ascorbic acid would be helpful in removing minerals - but then if you rinse with mineral-laden water you begin the cycle anew. An occasional chelating rinse, followed up with conditioning and rinsing with distilled water might "re-set" your hair. For some people, that's enough. For others, it's not enough and hair continues to be unruly or dry or brittle. Continuing with distilled water rinses (just to rinse out conditioner) or even half distilled water and half tap water would be much softer water. Where I live, you can buy distilled water in recyclable gallon plastic jugs, or purchase a home distilling machine, which looks like a large coffee-maker. Best wishes - W
Hi,
ReplyDeleteSo I live in Jerusalem, where the water is very hard and imported products cost a pretty penny. Trying to figure out how to have nice hair again, as I do whenever I fly to my lovely slightly acidic soft water in my home country. I've tried the ACV rinse and it works beautifully, but I'm concerned with frequency: I dilute it 1 to 4 parts in water, but if I only use it once a month it means I have nice hair for 3 days out of a month. How frequent is it safe to use it? Is there anything I can add in to make it safer?
Thank you
Hello Luisa, If you make the vinegar mixture a little more dilute, you might be able to use it as often as once every 1-2 weeks if your hair is healthy and strong. But watch your hair carefully for signs of dryness if you use it that often. The pH of the mixture I posted is about 3.1, and it won't go much lower with more or less vinegar - but the concentration matters a great deal. More vinegar is more aggressive. Just like more sugar makes a food sweeter, or more hot pepper makes it hotter. Good luck! W
DeleteHi, thank you so much for your answer! I ordered a filter at amazon, and somehow it worked (maybe the hardness was not the main problem?) Either way, my hair is back to its usual self, but this is super useful to know! Thanks so much!
DeleteHi, this is an amazing blog, thank you for your expertise! I usually have long, highlighted hair which looks ash blonde and blends well with my natural grey as it grows out, so I don't end up with a line and can get away with colour-treating just 2 or 3 times a year. However, I moved into a "tiny house" a month ago on our new rural property and am distressed that my hair has turned orange! I mean, really orange. And literally after the first shower. Our water is from a drilled well, and the analysis from the water report we had done includes the following:
ReplyDeletepH: 8.1
Alkalinity, total (as CaCO3): 610
TDS: 730
Hardness, total (as CaCO3): 154
Calcium: 18.6
Iron: 0.181
Magnesium: 26.2
Manganese: 0.0773
I assume it's the iron that's causing the orange? Would a chelating shampoo with EDTA remove it? As the house is so small, we don't have any room for installing a water softener or even storing bottles of town water for me to wash my hair with. Any advice you can give me for how to get rid of - and stay rid of - this unwanted "foxy" hair would be hugely appreciated! Thank you so much! (And apologies if this got posted twice.)
Hello CaroB - I can relate. And you made me laugh out loud at your "foxy" hair! Well water with iron in it can do that, though yours isn't high enough to meet the standard for, "Seriously orange colored water," set by the Environmental Protection Agency. If your water is a funky orange color - then I read that wrong. Otherwise - I think the problem is the high alkalinity. That tends to turn light-colored hair reddish, and the presence of iron could make it more red/orange. You didn't list the units (ppm or mg/L) that your test was in, so I'm making an educated guess. The pH is higher than neutral - yay - that increases the amount of minerals in your hair. Your alkalinity is *high* which is pretty common for well water. Not only will that turn light-colored hair orange, it can also make hair susceptible to breakage. Alkalinity is another form of minerals in water, it's the form that cannot be removed by a water softener. Your water is moderately hard too - so your overall mineral load is really high.
DeleteA chelating shampoo with EDTA will help with "hardness" minerals, but not Alkalinity minerals. The chelation done by EDTA requires a positive charge, and alkalinity minerals don't have that. Alkalinity minerals tend to respond better to vinegar rinses or products like Malibu Hard Water Treatment (it's sold in packets of crystals). Ion makes one too -Ion Crystal Clarifying Treatment. The ingredients are almost the same as Malibu.
Vinegar rinses are cheap, and should be prepared with distilled water so there are no minerals. They can be done every wash-day if you don't make them too concentrated and use a good conditioner or oil treatments. You could even do a distilled water rinse after so you're not adding minerals. -- Another thing you can do is to soak your hair with distilled water before showering - so the first water it absorbs isn't quite so mineral-laden. Swimmers do this to try to absorb less chlorinated water. I know you said no distilled water - but mixing up 1 cup for a rinse and soaking your hair with 1 cup's worth before a shower isn't a whole lot of distilled water...
Reverse osmosis is one way to deal with this problem, but that can take up an amount of space you probably don't have under your sink. I would probably store a bottle of distilled water outside if possible. Maybe in a camp-shower or solar shower so it can warm in the sun and be hang up out of the way. Using penetrating oil treatments and conditioning before washing both would help protect hair against "soaking up" minerals to some degree. I hope that helps - W
Amazing blog. Thank you sooo much!! This is my question: I would like to make a DIY mild chelating shampoo for my 4 year old daughter. Water is OK in our area but she goes to the swimming pool once a week and sometimes we travel to hard water areas.
ReplyDeleteAfter reading your replies about EDTA, I was thinking about adding phytic acid (or sodium gluconate) to a regular shampoo, how much would be OK?
Hello E77 - Phytic acid/Sodium phytate powder needs to be used in small amounts. The pH of a 1% solution is 12, which is much too high to be safe for eyes and skin. The recommended use is 0.05 to 0.5% (or 0.05 to 0.5 grams per 100 ml product). And then the product needs to have the pH adjusted to about 6 so it is safe for skin and eyes (and hair). I would probably skip this one!
DeleteSodium gluconate is less likely to change the pH of your product. It is used at less than 1%, so a scale that reads out 2 decimal points is necessary. It may take some time and stirring to dissolve thoroughly.
Citric acid makes a good chelating rinse, you can add 1/16 teaspoon to 1 cup water and leave that on for a minute or so. The pH of this is on the low side, it will sting eyes and cuts or scrapes and should be kept away from the eyes, nose or mouth. I use that rinse - after washing and before conditioning. Good luck and safe-formulating. -W
I have hair colored to cover the gray and highlights. My hair color gets warmer after a few weeks even though we have a water softener and a whole house water filter for our well. Even when I lived other places with city water and a water softener, the same results occurred. The brand of coloring or stylist techniques have never been able to keep my hair color neutral or ash for more than four weeks. Is this caused by absorption of minerals? How can I clarify the minerals without washing out the color that I paid to have done?
ReplyDeleteHello, my whole family suffers from super tangled, dry, itchy scalp hair and has let to lots of thinning and now hair loss in all of us. We have very hard well water and the landlord has a salt water softener on the house but he has the setting too low on the water softener for the hardness grains. The TDS is 650 in a water report i had done. would a showerstick work for this problem? What is best for our hair, chelating shampoos, citric acid rinses or vinegar rinses? I need help. Thank you
ReplyDeleteCynthia